Study Tip: A Level Electrochemistry
04/11/25
Top Tips for A-Level Electrochemistry: Understanding Half Cells the Smart Way
Electrochemistry can be one of those A-Level Chemistry topics that students either love or dread — but it doesn’t have to be confusing. Here are two top tips from The Chemistry Coach A.I. to help you master how half-cells combine and how to remember which side does what.
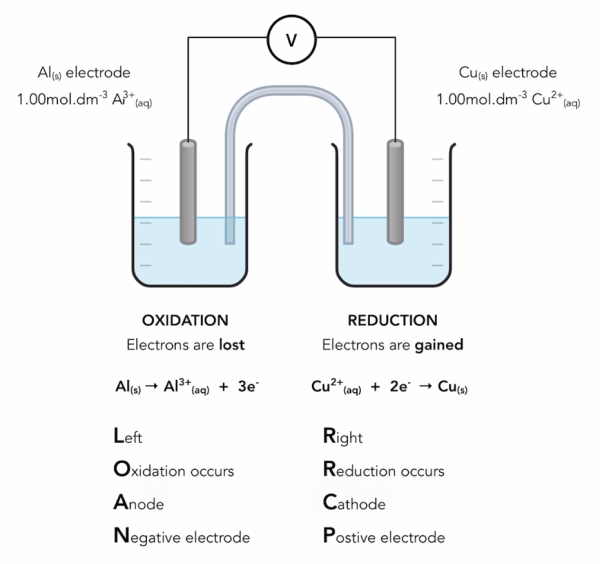
Tip 1: Always Put the Most Negative Half Cell on Top
Whenever you’re combining two half-cells, start by writing out the two half-equations — but put the most negative one on top.
For example:
Aluminium (Al) has a more negative electrode potential than copper (Cu).
So, aluminium goes on top, and copper goes below.
Once they’re arranged, you can apply the anti-clockwise rule to predict what happens when these two half-cells are connected:
-
Aluminium loses electrons → it’s oxidised.
-
Copper gains electrons → it’s reduced.
Simple rule:
???? Most negative on top, anti-clockwise gives oxidation → reduction.
Tip 2: Remember the “L.O.A.N.” Convention
When it comes to drawing or interpreting electrochemical cells, conventions matter — and there’s one you can always rely on.
On the left-hand side, you’ll find the half-cell where oxidation happens.
This side is known as the anode, and it’s the negative electrode.
To make it stick, remember the word L.O.A.N.
Left — Oxidation — Anode — Negative
So, whenever you see an electrochemical cell diagram, you can confidently identify what’s happening just by checking the left-hand side.
In Short
-
Write the most negative half-cell on top.
-
Apply the anti-clockwise rule: oxidation → reduction.
-
Use L.O.A.N. to remember the left side conventions.
Follow these steps, and electrochemistry becomes far less daunting — especially when you practise with real exam questions.
Want more step-by-step Chemistry help?
Chat with Chemistry Coach A.I. — your 24/7 personal tutor that knows the AQA, OCR A and WJEC specifications down to the last letter. It can guide you through electrochemistry, equations, and every tricky concept the same way an expert tutor would.





